Justice Pending for Patients Who Underwent Experimental Heart Surgeries Without Consent
Illinois Supreme Court Reviewing Request for Supervisory Order
NEWS PROVIDED BY
Most Sacred Heart of Jesus Cardiology and Valvular Institute
Sept. 29, 2021
CHICAGO, Sept. 29, 2021 /Standard Newswire/ -- More than 1,000 patients who underwent experimental heart surgeries without their consent and without FDA authorization finally may get justice.
The Illinois Supreme Court accepted a motion on Sept. 17, 2021 that would compel the Illinois Attorney Registration and Disciplinary Commission to submit life-saving medical evidence to Northwestern University's general counsel. That, in turn, would require the university's human subject research protections program to inform those patients.
Northwestern University attorney Stephanie Graham and Ann Adams, vice president for research, have stated that the "Myxo Ring" heart device was "FDA approved" since 2004. The device manufacturer's attorney David Jensen of Eichorn and Eichorn, however, stated the second version of the Edwards Lifesciences device was not approved until April 2009.
Transferring that evidence to Northwestern University would start the 14-year process of informing the hundreds of patients who never were informed of the investigational nature of the device and the experimental heart surgery, under the federal reporting regulations 45CFR46 and 21CFR50.
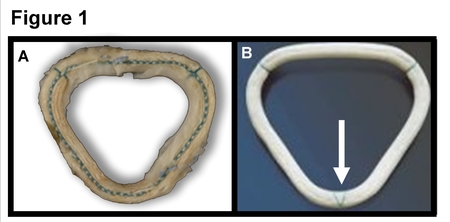
Dr. Nalini Rajamannan, a Mayo Clinic Trained Cardiologist has been advocating on behalf of these patients, who are unaware that they received unapproved FDA devices. Since 2006, more than 1000 patients have undergone the experimental heart surgery without consent. The Daily Northwestern newspaper reported on one patient's death in 2018. Other publications have reported over the years on other patients' complications. But the full scope of harm can't be documented until all patients are made aware of the issue. Figure 1, the Investigational "Myxo ring," Figure 2, the second version, the Detlogix ring approved by the FDA in April 2009.
In reviewing his own health records in 2018, Dr. Al Edwards discovered he had had a heart attack 11 years earlier and not been told. "I requested more information from the university office of human subject protections and I received the following letter stating, 'we consider this matter closed.'"
In the scientific article regarding the experimental heart surgeries published in the Journal of Thoracic and Cardiovascular Surgery, by the American Association of Thoracic Surgeons, the authors confirmed that patients waived their "patients' rights to know" regarding the study they were enrolled in as of November 2020.
The FDA requested that Northwestern University retroactively inform patients—dating back to November 2008—that their registry data was used to publish the initial "first in human testing" of the Myxo Ring. Instead of reporting the testing, Northwestern Hospital's president and CEO Dean Harrison in January 2009 sent a letter to Edwards and other patients who had received the Myxo ring. It stated that "we do not consider this device to be experimental."
This information was published by the Chicago Tribune in May 2011. In the same story, Dr. Jeff Shuren, head of FDA medical device branch, confirmed the opposite, saying "If a device is supposed to get cleared by the agency first before you're marketing it, we consider that investigational."
As of today, the patients have not been informed. The Illinois Supreme Court now has jurisdiction over this process.
SOURCE Most Sacred Heart of Jesus Cardiology and Valvular Institute
CONTACT: Dr. Nalini M. Rajamannan, 312-498-9496, nrajamannan@gmail.com

NEWS PROVIDED BY
Most Sacred Heart of Jesus Cardiology and Valvular Institute
Sept. 29, 2021
CHICAGO, Sept. 29, 2021 /Standard Newswire/ -- More than 1,000 patients who underwent experimental heart surgeries without their consent and without FDA authorization finally may get justice.
The Illinois Supreme Court accepted a motion on Sept. 17, 2021 that would compel the Illinois Attorney Registration and Disciplinary Commission to submit life-saving medical evidence to Northwestern University's general counsel. That, in turn, would require the university's human subject research protections program to inform those patients.
Northwestern University attorney Stephanie Graham and Ann Adams, vice president for research, have stated that the "Myxo Ring" heart device was "FDA approved" since 2004. The device manufacturer's attorney David Jensen of Eichorn and Eichorn, however, stated the second version of the Edwards Lifesciences device was not approved until April 2009.
Transferring that evidence to Northwestern University would start the 14-year process of informing the hundreds of patients who never were informed of the investigational nature of the device and the experimental heart surgery, under the federal reporting regulations 45CFR46 and 21CFR50.
Dr. Nalini Rajamannan, a Mayo Clinic Trained Cardiologist has been advocating on behalf of these patients, who are unaware that they received unapproved FDA devices. Since 2006, more than 1000 patients have undergone the experimental heart surgery without consent. The Daily Northwestern newspaper reported on one patient's death in 2018. Other publications have reported over the years on other patients' complications. But the full scope of harm can't be documented until all patients are made aware of the issue. Figure 1, the Investigational "Myxo ring," Figure 2, the second version, the Detlogix ring approved by the FDA in April 2009.
In reviewing his own health records in 2018, Dr. Al Edwards discovered he had had a heart attack 11 years earlier and not been told. "I requested more information from the university office of human subject protections and I received the following letter stating, 'we consider this matter closed.'"
In the scientific article regarding the experimental heart surgeries published in the Journal of Thoracic and Cardiovascular Surgery, by the American Association of Thoracic Surgeons, the authors confirmed that patients waived their "patients' rights to know" regarding the study they were enrolled in as of November 2020.
The FDA requested that Northwestern University retroactively inform patients—dating back to November 2008—that their registry data was used to publish the initial "first in human testing" of the Myxo Ring. Instead of reporting the testing, Northwestern Hospital's president and CEO Dean Harrison in January 2009 sent a letter to Edwards and other patients who had received the Myxo ring. It stated that "we do not consider this device to be experimental."
This information was published by the Chicago Tribune in May 2011. In the same story, Dr. Jeff Shuren, head of FDA medical device branch, confirmed the opposite, saying "If a device is supposed to get cleared by the agency first before you're marketing it, we consider that investigational."
As of today, the patients have not been informed. The Illinois Supreme Court now has jurisdiction over this process.
SOURCE Most Sacred Heart of Jesus Cardiology and Valvular Institute
CONTACT: Dr. Nalini M. Rajamannan, 312-498-9496, nrajamannan@gmail.com




 Sign Up to Receive Press Releases:
Sign Up to Receive Press Releases: